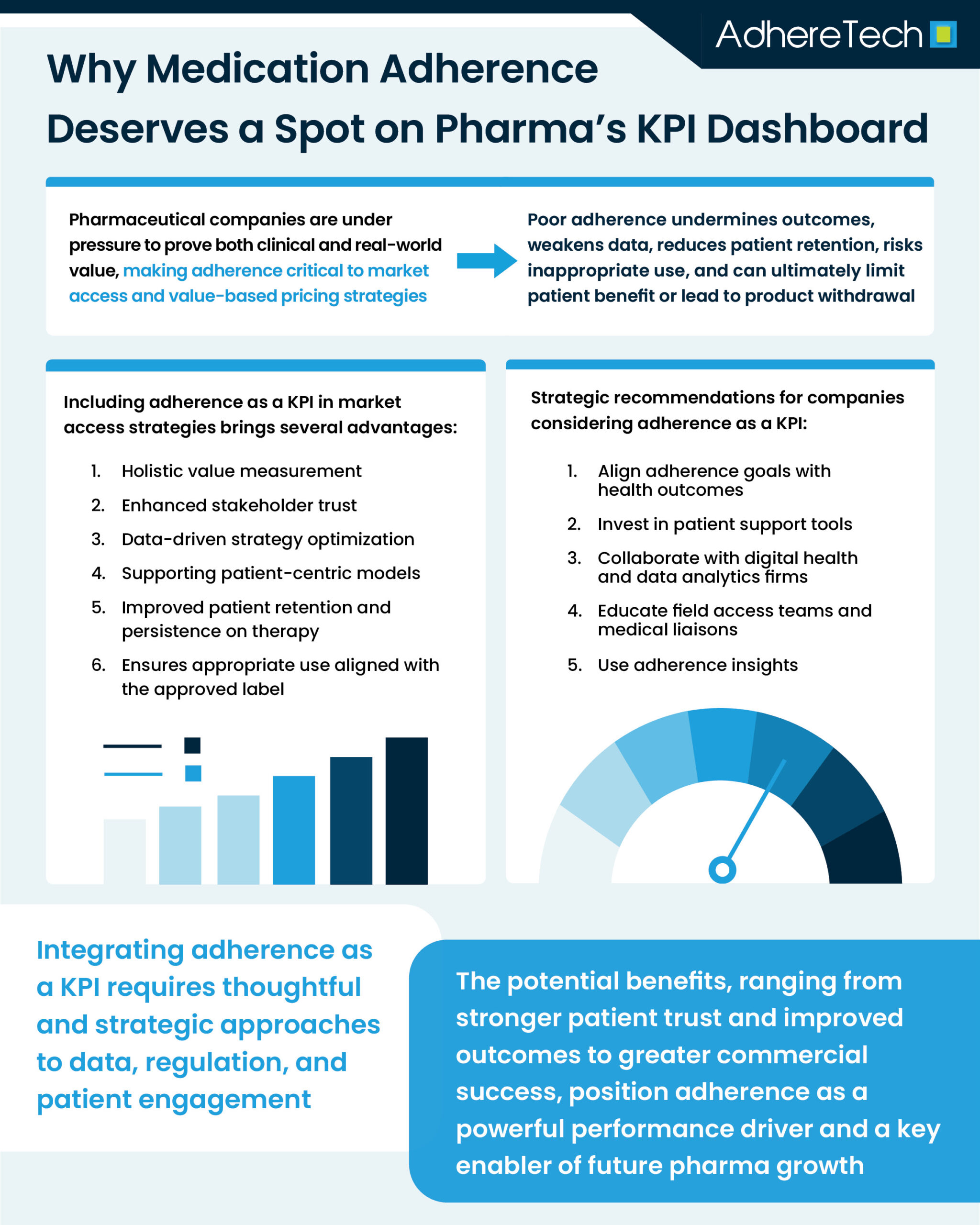
In today’s competitive and outcomes-driven healthcare environment, pharmaceutical companies are under growing pressure to demonstrate not only the efficacy and safety of their products in each phase of a clinical trial, but also their real-world value. Market access strategies have evolved to address this need, often incorporating health economics, real-world evidence, and patient-centric models. One question gaining traction is whether medication adherence — the extent to which patients take their medications as prescribed1 — should be considered a key performance indicator (KPI) in market access strategies.2-4
From a market access perspective, the goal is to ensure that therapies not only reach patients, but also achieve the intended health and economic outcomes.5 If patients do not take their medication as intended, this can result in a distortion of pharmacometric, with the drug underperforming or prescriptions being filled infrequently. Consequently, sponsors can lose the opportunity for return on investment, which is often tens of millions, and patients may fail to receive the drug’s true benefits, resulting in eventual withdrawal of the market product.6
This raises a fundamental question: If a medication does not deliver its full expected efficacy due to poor adherence, should pharmaceutical companies bear some responsibility, and track adherence as a strategic performance metric?
Why Medication Adherence Matters for Pharma
Health systems and payers are increasingly shifting toward value-based pricing and outcome-based reimbursement models, which reward pharmaceutical companies according to how well a drug performs in real-world use rather than solely in clinical trials.7 Because adherence directly impacts whether a drug is able to demonstrate its full efficacy , particularly for therapies that only deliver their full benefit when taken consistently, pharmaceutical companies have a clear incentive to promote and support adherence as part of a broader value demonstration strategy.8 Low adherence rates in observational studies or utilization of measures that grossly overestimate adherence in clinical trials can dilute the data, making it impossible to determine the true effectiveness of a treatment, weakening health economic evaluations and jeopardizing patient health outcomes.10
Beyond the regulatory and reimbursement implications, adherence has a direct commercial impact. Poor adherence can reduce drug utilization, undermine brand loyalty, and lead patients to discontinue treatment, switch therapies, or report low satisfaction,11 all factors that negatively influence sales forecasts, formulary positioning, and market share. By treating adherence as a core performance indicator, companies can not only improve data quality regarding new treatments, but also encourage sustained engagement, improve patient health outcomes, and generate higher returns on investment.
The Case for Making Adherence a KPI
Including adherence as a KPI in market access strategies brings several advantages:
1. Holistic Value Measurement
Traditional KPIs in market access often focus on reimbursement, pricing, and formulary placement.7 However, these factors do not directly guarantee successful patient outcomes. By incorporating adherence, companies can align commercial success with therapeutic success, creating a more holistic performance framework that prioritizes patients.
2. Enhanced Stakeholder Trust
Payers and providers are more likely to collaborate with manufacturers that demonstrate a commitment to improving health outcomes.12 Tracking adherence shows that the company is not only interested in selling a product but is also invested in ensuring it truly improves patient outcomes.
3. Data-Driven Strategy Optimization
Adherence data can inform multiple aspects of strategy, from patient segmentation and support programs to communication with healthcare professionals. Companies can identify adherence barriers in specific populations and tailor their market access initiatives accordingly to ensure the results of clinical trial treatments translate to tangible, real-world results.
4. Supporting Patient-Centric Models
Regulators and healthcare systems are moving toward more patient-centered care models. By prioritizing adherence and using evidence-based monitoring methods, pharmaceutical companies signal alignment with this broader shift and demonstrate that patient experience and engagement are integral to their strategy.
Challenges and Considerations
Despite its potential, integrating adherence as a KPI is not without obstacles. While pharmaceutical companies can provide tools and education, adherence is shaped by numerous factors (including socioeconomic conditions, healthcare provider relationships, and individual patient behavior)13 making it a complex, shared responsibility that risks being oversimplified when treated as a KPI. Capturing accurate adherence data requires real-time monitoring that eliminates social and desirability biases.,14 However, this may present technical and privacy challenges, particularly when considering interoperability and regulatory compliance requirements such as GDPR.15
Measurement is further complicated by the lack of standardization, as adherence can be calculated in different ways — such as proportion of days covered (PDC), medication possession ratio (MPR), or persistence — making it difficult to benchmark across therapeutic areas or geographies. Finally, although adherence initiatives can improve outcomes, their return on investment is not always clear-cut, requiring companies to weigh the costs of intervention programs against the potential gains in drug performance or market share.
Real-World Examples and Innovation
Several pharmaceutical companies have already started incorporating adherence support into their market strategies. For example, Pfizer has implemented a range of patient support programs aimed at enhancing adherence, particularly within oncology and rare disease treatments, where medication regimens are often complex, costly, and emotionally taxing. These programs typically combine financial assistance, insurance navigation, and personalized care coordination to reduce barriers that could lead to treatment interruptions. In oncology, for example, Pfizer’s initiatives include nurse-led patient education on managing side effects, regular check-ins to monitor progress, and tools for scheduling and medication reminders, helping patients remain on track with their prescribed therapies.16 In rare diseases, where treatments are often lifelong and patient populations are small, Pfizer has emphasized tailored, high-touch support models that address both clinical and psychosocial needs, including access to genetic counseling, coordination with specialty pharmacies, and facilitation of diagnostic testing17. By integrating these services, Pfizer not only aims to improve medication adherence but also to build trust, increase patient engagement, and support better health outcomes, recognizing that the success of advanced therapies depends heavily on consistent, uninterrupted treatment. However, a key limitation remains: while such programs can monitor prescription refills, scheduling, and patient engagement, they cannot determine with certainty whether patients are actually taking their medications as prescribed—a gap in industry knowledge that continues to challenge accurate adherence measurement. Even so, these programs provide Pfizer with valuable real-world insights into patient behavior and treatment challenges, informing future program design and therapeutic innovation.
These examples show that adherence is not just a theoretical KPI — it is increasingly becoming a practical performance driver for forward-thinking pharma companies.
Strategic Recommendations
If pharma companies are considering incorporating adherence as a KPI in market access strategies, they should:
- Align adherence goals with health outcomes in payer and provider contracts.
- Invest in patient support tools, such as reminder systems, smart devices, and adherence support from healthcare providers.
- Collaborate with digital health and data analytics firms to capture and interpret adherence data.
- Educate field access teams and medical liaisons on how to discuss adherence with stakeholders.
- Use adherence insights to segment markets and tailor interventions for high-risk populations.
Adherence is a critical driver of real-world therapeutic success, yet it remains underutilized in many pharma market access strategies. As value-based healthcare continues to evolve, integrating adherence as a KPI is not only logical, but necessary.
By tracking and supporting adherence, pharmaceutical companies can demonstrate greater value to payers, deliver better outcomes for patients, and build stronger, more sustainable market access strategies. While there are challenges in measurement and implementation, the potential rewards — in trust, outcomes, and commercial success — make adherence a compelling KPI for the future of pharma.
References
“Rare Disease Awareness, Support & Advocacy | Pfizer.” Pfizer.com, 2025, www.pfizer.com/about/responsibility/patient-advocacy-engagement-putting-patients-first/advocacy. Accessed 15 Aug. 2025.cl.ac.uk/prospective-students
Brown, Marie T., and Jennifer K. Bussell. “Medication Adherence: WHO Cares?” Mayo Clinic Proceedings, vol. 86, no. 4, Apr. 2011, pp. 304–314, https://doi.org/10.4065/mcp.2010.0575.
Claritas Rx. “Essential KPIs for Your Pharmaceutical Market Access Strategy.” ClaritasRx, 8 Nov. 2022, www.claritasrx.com/blog/market-access-strategy/? Accessed 15 Aug. 2025.
AllazoHealth. “Maximize Medication Adherence by Defining the Right KPI Metrics.” AllazoHealth, 9 Mar. 2023, allazohealth.com/resources/establishing-kpis-for-medication-adherence/? Accessed 15 Aug. 2025.
MarketResearch.com. “Medication Adherence.” Marketresearch.com, June 2025, www.marketresearch.com/Global-Industry-Analysts-v1039/Medication-Adherence-41260226/. Accessed 15 Aug. 2025.
Kleinsinger, Fred. “The Unmet Challenge of Medication Nonadherence.” The Permanente Journal, vol. 22, no. 18-033, 5 July 2018, www.ncbi.nlm.nih.gov/pmc/articles/PMC6045499/, https://doi.org/10.7812/tpp/18-033.
Le Flohic, Elise, et al. “The Impacts of Undetected Nonadherence in Phase II, III and Post-Marketing Clinical Trials: An Overview.” British Journal of Clinical Pharmacology, vol. 90, no. 8, Aug. 2024, pp. 1984–2003, pubmed.ncbi.nlm.nih.gov/38752447/, https://doi.org/10.1111/bcp.16089.
Fatoye, Clara, et al. “Conceptualisation and Role of Market Access in Pharmaceutical Industry: A Scoping Review.” Journal of Market Access & Health Policy, vol. 12, no. 2, 1 June 2024, pp. 81–99, www.mdpi.com/2001-6689/12/2/7, https://doi.org/10.3390/jmahp12020007.
Ntais, Christos, et al. “Managing Pharmaceutical Costs in Health Systems: A Review of Affordability, Accessibility and Sustainability Strategies.” Journal of Market Access & Health Policy, vol. 12, no. 4, 10 Dec. 2024, pp. 403–414, pmc.ncbi.nlm.nih.gov/articles/PMC11677551/pdf/jmahp-12-00031.pdf, https://doi.org/10.3390/jmahp12040031.
Viswanathan, Meera, et al. “Interventions to Improve Adherence to Self-Administered Medications for Chronic Diseases in the United States: A Systematic Review.” Annals of Internal Medicine, vol. 157, no. 11, 4 Dec. 2012, pp. 785–95, www.ncbi.nlm.nih.gov/pubmed/22964778, https://doi.org/10.7326/0003-4819-157-11-201212040-00538.
Cutler, Rachelle Louise, et al. “Economic Impact of Medication Non-Adherence by Disease Groups: A Systematic Review.” BMJ Open, vol. 8, no. 1, 2018, p. e016982, bmjopen.bmj.com/content/bmjopen/8/1/e016982.full.pdf, https://doi.org/10.1136/bmjopen-2017-016982.
Burns, Leah, et al. “Real-World Evidence for Regulatory Decision-Making: Guidance from around the World.” Clinical Therapeutics, Feb. 2022, https://doi.org/10.1016/j.clinthera.2022.01.012.
Abualbishr Alshreef, et al. “Statistical Methods for Adjusting Estimates of Treatment Effectiveness for Patient Nonadherence in the Context of Time-To-Event Outcomes and Health Technology Assessment: A Systematic Review of Methodological Papers.” Medical Decision Making, vol. 39, no. 8, 24 Oct. 2019, pp. 910–925, pmc.ncbi.nlm.nih.gov/articles/PMC6900590/, https://doi.org/10.1177/0272989×19881654. Accessed 9 Feb. 2025.
Hill-McManus, Daniel, et al. “Impact of Non-Adherence and Flare Resolution on the Cost-Effectiveness of Treatments for Gout: Application of a Linked Pharmacometric/Pharmacoeconomic Model.” Value in Health, vol. 21, no. 12, 26 July 2018, pp. 1373–1381, https://doi.org/10.1016/j.jval.2018.06.002. Accessed 18 May 2025.
Etges, Ana Paula Beck de Silva, et al. “Value-Based Reimbursement as a Mechanism to Achieve Social and Financial Impact in the Healthcare System.” Journal of Health Economics and Outcomes Research, vol. 10, no. 2, 31 Oct. 2023, pp. 100–103, jheor.org/article/89151-value-based-reimbursement-as-a-mechanism-to-achieve-social-and-financial-impact-in-the-healthcare-system, https://doi.org/10.36469/001c.89151.
Baryakova, Tsvetelina H., et al. “Overcoming Barriers to Patient Adherence: The Case for Developing Innovative Drug Delivery Systems.” Nature Reviews Drug Discovery, vol. 22, no. 22, 27 Mar. 2023, pp. 1–23, https://doi.org/10.1038/s41573-023-00670-0.
“Pfizer Harnesses Digital Health Solutions to Improve Patients’ Lives Licing with Breast Cancer.” Www.pfizer.com, www.pfizer.com/sites/default/files/investors/financial_reports/annual_reports/2022/story/harnessing-digital-health-solutions-to-improve-patients-lives/.