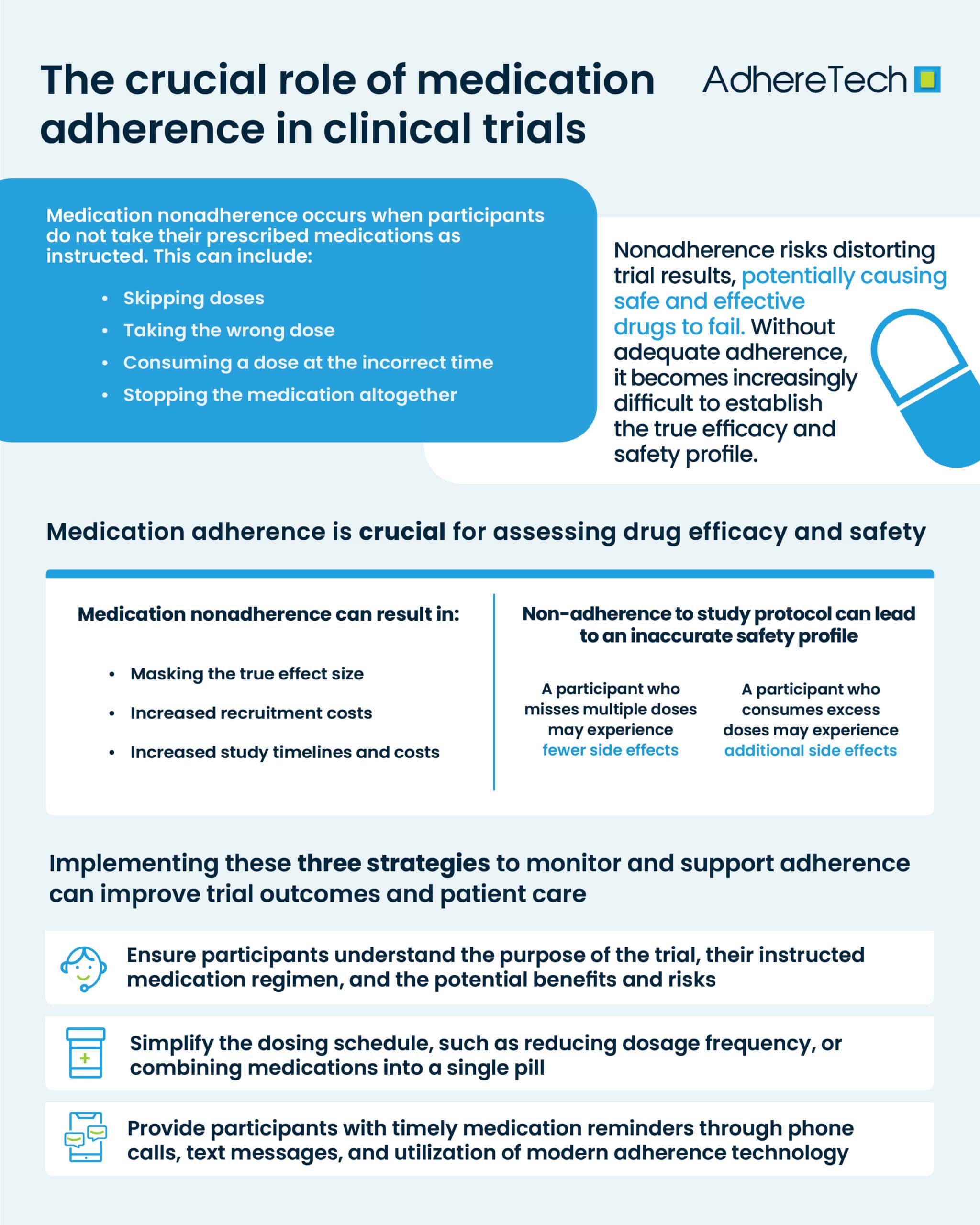
What Is Medication Nonadherence
Medication nonadherence occurs when participants do not take their prescribed medications as instructed1. This can include skipping doses, taking the wrong dose, consuming a dose at the incorrect time, or stopping the medication altogether. In clinical trials, nonadherence can be particularly problematic, as it can skew results, causing studies for potentially safe and efficacious drugs to fail, depriving patients of what could be a badly needed new medicine2.
One of the most significant risks of medication nonadherence is the distortion of trial results. Clinical trials are designed to assess the effectiveness and safety of a particular drug3, and these assessments rely on participants following the exact treatment regime prescribed. When participants fail to adhere to their instructed medication schedule, without adequate adherence measures, it becomes impossible to determine whether the observed effects (or lack thereof) are due to the medication itself or nonadherence.
Importance of Medication Adherence
Medication adherence is not only essential for measuring effectiveness, but also for evaluating the safety profile of a drug. Nonadherence may lead to participants receiving suboptimal or excessive doses of the treatment, potentially altering the observed side effects4. For instance, a participant who misses multiple doses of a medication might experience fewer side effects than someone who adheres strictly to the treatment plan. Meanwhile, a participant who consumes excess of their medication before a trial visit, may experience immense side effects. In both scenarios this would lead to an inaccurate assessment of the drug’s safety profile.
Medication nonadherence can bias clinical trial results5, which in turn, can affect the generalizability of the findings. Trials are designed to reflect how a treatment would perform in a broad population, but when nonadherence skews the data, it becomes exceedingly difficult to determine how the treatment would fare in real-world conditions. Futhermore, as there is less oversight of patients outwith clinical trial settings, medication adherence is actually likely to be lower in real-world settings. This can reduce the overall external validity of the trial, meaning the findings may not accurately represent the drug’s true potential.
Medication non-adherence can also significantly impact the timelines, and costs of clinical trials. When participants fail to take their prescribed medications as directed, it can lead to unreliable data, making it difficult to assess the true effectiveness of the treatment being tested. This can result in skewed results, potentially causing delays in the trial or the need for additional studies to obtain accurate data. Furthermore, non-adherence can extend the timeline of a trial, as researchers may need to account for the lack of compliance by recruiting more participants or adjusting the study design. From a financial perspective, medication non-adherence increases overall costs and timelines of clinical trials, as it may require additional resources for monitoring, recruitment, and follow-up, all of which strain the trial budget and delay the path to regulatory approval.
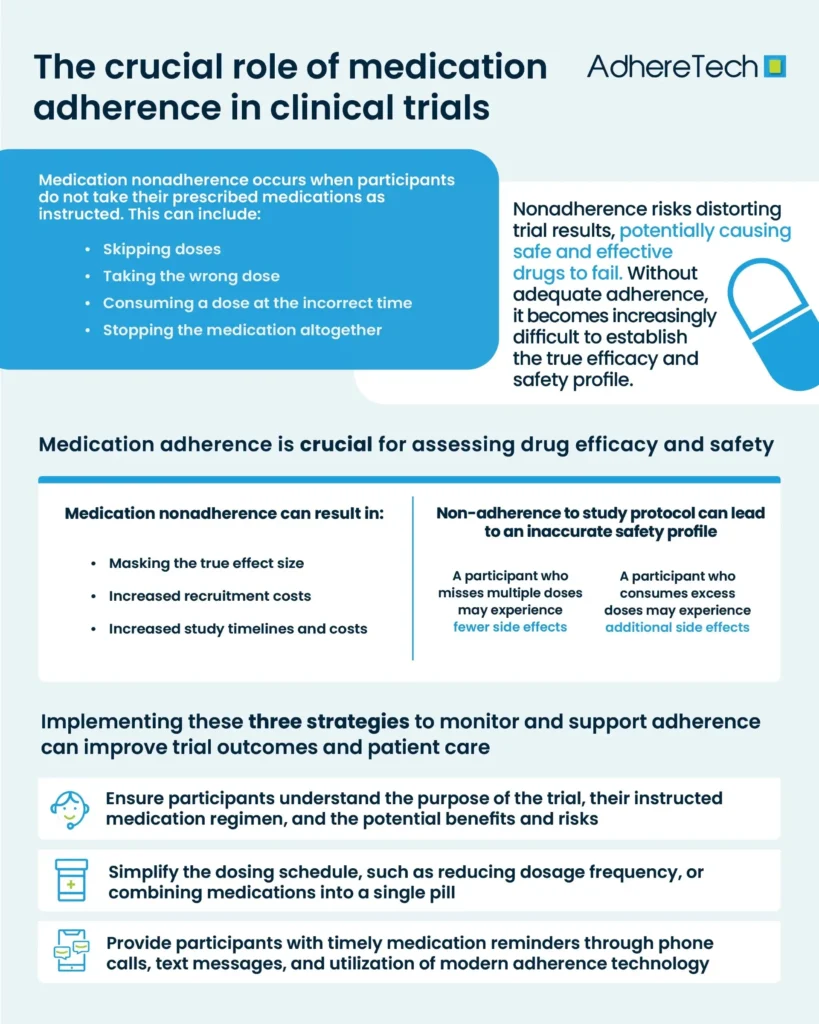
How to Improve Medication Adherence
Medication adherence is a critical factor in ensuring the validity and reliability of trial outcomes. Yet, despite its significance, nonadherence remains a pervasive issue in many clinical trials4, impacting not only the success of the research but also the safety and well-being of participants. In order to mitigate the issue, researchers and healthcare providers should:
- Ensure participants fully understand the purpose of the trial, their instructed medication regimen, and the potential benefits and risks.
- Simplify the dosing schedule, such as reducing the frequency of doses or combining medications into a single pill, making it easier for participants to stick to their regime.
- Provide participants with medication reminders through phone calls, text messages, and utilisation of modern adherence technology.
It is also essential to ensure that the adoption of these strategies fit within the patient’s routine, and do not create additional burden, which would have the potential to increase non-adherence and mask the true effect of the drug. Effectively incorporating these three strategies to monitor, encourage, and support adherence maximizes the potential for successful outcomes in trials and better patient care.
References
- Cramer, J. A., Roy, A., Burrell, A., Fairchild, C. J., Fuldeore, M. J., Ollendorf, D. A., & Wong, P. K. (2008). Medication compliance and persistence: terminology and definitions. Value in Health: The Journal of the International Society for Pharmacoeconomics and Outcomes Research, 11(1), 44–47. https://doi.org/10.1111/j.1524-4733.2007.00213.x
- Eliasson, Lina, et al. “How the EMERGE Guideline on Medication Adherence Can Improve the Quality of Clinical Trials.” British Journal of Clinical Pharmacology, vol. 86, no. 4, 28 Feb. 2020, pp. 687–697, https://doi.org/10.1111/bcp.14240.
- National Institute on Aging. “What Are Clinical Trials and Studies?” National Institute on Aging, 22 Mar. 2023, www.nia.nih.gov/health/clinical-trials-and-studies/what-are-clinical-trials-and-studies.
- Shiovitz, Thomas M., et al. “Mitigating the Effects of Nonadherence in Clinical Trials.” The Journal of Clinical Pharmacology, vol. 56, no. 9, 22 Jan. 2016, pp. 1151–1164, https://doi.org/10.1002/jcph.689.
- Murali, Karumathil M., et al. “Medication Adherence in Randomized Controlled Trials Evaluating Cardiovascular or Mortality Outcomes in Dialysis Patients: A Systematic Review.” BMC Nephrology, vol. 18, no. 1, 31 Jan. 2017, p. 42, pubmed.ncbi.nlm.nih.gov/28143438/, https://doi.org/10.1186/s12882-017-0449-1.